
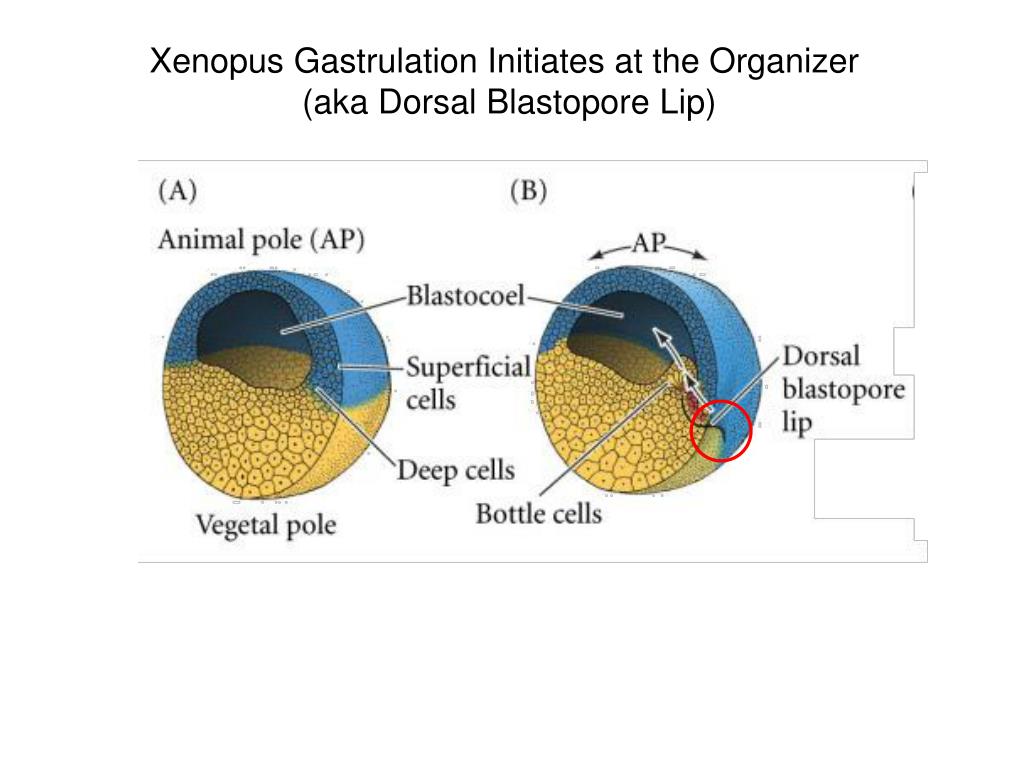
The processes of bottle cell formation, blastopore lip formation, and involution all begin dorsally and spread laterally, e.g. Rather than being a static group of cells, the blastopore lip is a dynamic annular mass of cells, composed of both superficial epithelia and deep mesenchymal cells that transiently reside in the lip as they move into the embryo. The bending of the dorsal marginal zone as involution progresses forms the blastopore lip.
Involution starts when dorsal marginal zone cells adjacent to the bottle cells and deepening groove move inward. Gastrulation begins as pigment accumulates at the apical face of forming bottle cells. Bottle cell constriction encircles the large yolky cells of the vegetal endoderm.
#BLASTOPORE LATERLA PATCH#
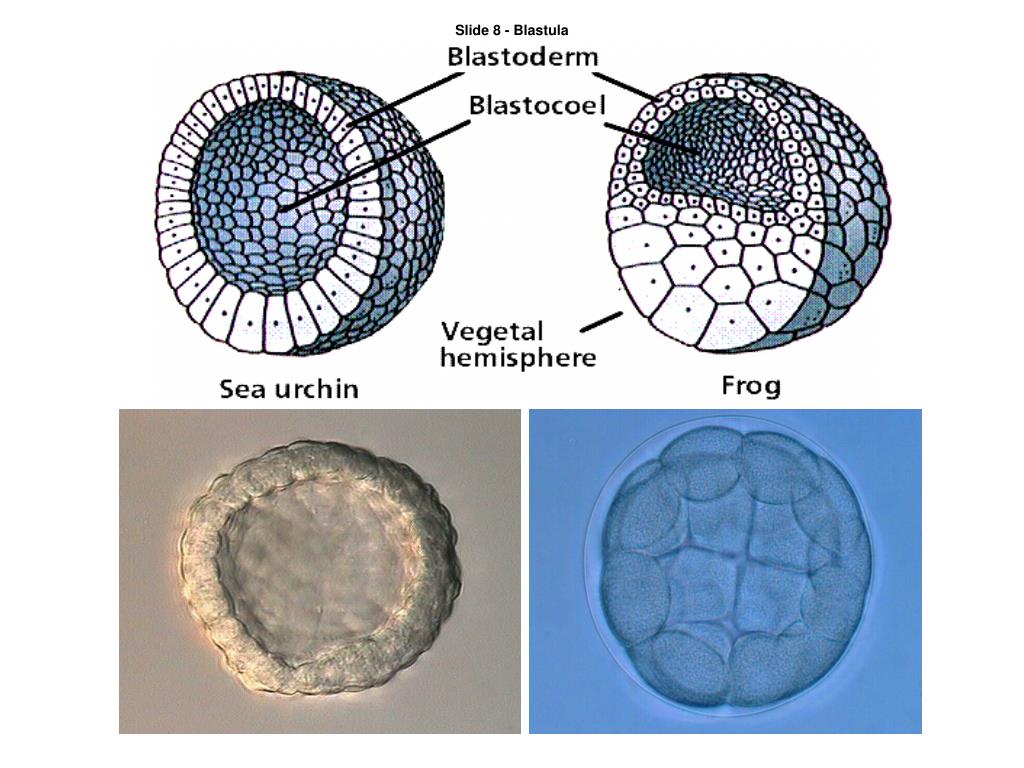
Gastrulation begins when a patch of epithelial cells known as bottle cells apically constrict and lengthen along their apical basal axis to create a local invagination that ultimately forms the anterior-most end of the future archenteron. During gastrulation, large regions on the surface of the embryo involute and pass into the embryo while the remaining surface tissues expand to enclose the embryo.
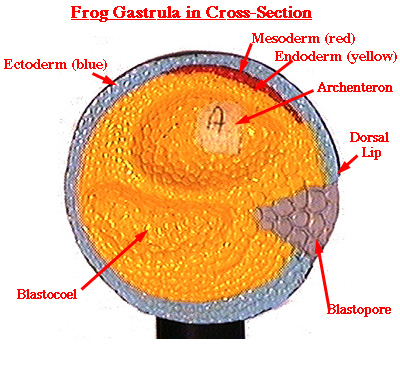
Tissue-scale mechanics are especially important during the large scale rearrangements of the germ layers during amphibian gastrulation ( Keller et al., 2000). These programs coordinate cell behaviors and drive morphogenetic movements, but also encode cell responses to variations in both the local microenvironment as well as to larger scale environmental cues. Developmental programs regulate mechanical processes by regulating cell motility, cell shape, and cell adhesion through direct genetic control as well as through biochemical and mechanical signaling pathways ( Beloussov et al., 1988 Discher et al., 2005 Orr et al., 2006). By tailoring force production to material properties the embryo is able to facilitate large scale tissue movements necessary to organize the three prospective germ layers during gastrulation ( Davidson, 2011 Moore et al., 1995). Morphogenesis relies on cell- and tissue-level control of mechanical properties and highly regulated, spatio-temporally controlled forces to dynamically reshape tissues ( Heisenberg and Bellaiche, 2013 Holtfreter, 1943, 1944 Koehl, 1990 Lecuit et al., 2011). Cell shape and F-actin alignment analyses reveal different local mechanical environments in regions around the blastopore, which was reflected by the strain rate maps. Interestingly F-actin was consistently oriented toward the blastopore lip in dorsal and lateral cells, but oriented parallel to the lip in ventral regions. The F-actin network is organized differently in each region with the highest percentage of alignment occurring in the lateral region. In contrast, cells lateral and ventral to the blastopore are less polarized and have tortuous cell boundaries. Cells dorsal to the blastopore, which are fated to become neural plate ectoderm, are polarized and have straight boundaries. Strain rate mapping of the dorsal, ventral and lateral epithelial cells proximal to the blastopore reveals changing patterns of strain rate throughout closure. During this time course, the embryo also stiffens 1.5 fold. We find that the embryo generates a ramping magnitude of force until it reaches a peak force on the order of 0.5 μ Newtons. We describe the mechanics of blastopore closure at multiple scales and in different regions around the blastopore by characterizing large scale tissue deformations, cell level shape change and subcellular F-actin organization and by measuring tissue force production and structural stiffness of the blastopore during gastrulation. These forces are tuned to generate sustained blastopore closure throughout the course of gastrulation. Blastopore closure in the amphibian embryo involves large scale tissue reorganization driven by physical forces.


 0 kommentar(er)
0 kommentar(er)
